Constituting the innermost layer of the amniotic cavity, the amniotic membrane consists of a single layer of cuboidal epithelial cells firmly adhered to a thick basement membrane, which is in turn attached to an avascular stromal layer. The cells in these layers produce biological factors and mediators that contribute to the therapeutic benefits of the amniotic membrane. The amniotic membrane is also thin, lightweight, and elastic making it suitable for a range of therapeutic applications.
Dehydrated amniotic membrane has a long clinical history in managing diabetic wounds, burns, and ocular repair. Research has shown that the membrane contains a variety of beneficial components including growth factors, heavy chain-hyaluronic acid, and a scaffold which all reinforce its application as a protective covering.
SurGraft® is a dehydrated amniotic membrane sheet produced using minimal manipulation. This process preserves the membrane to maintain its therapeutic properties while preparing it for shelf-stable storage, and flexible use in a variety of treatment applications.
SurGraft® is thoroughly tested for safety in clinical procedures. We pride ourselves on safety standards that exceed regulatory requirements by performing additional serological tests missed by our competitors.
SurGraft® is a dehydrated amniotic membrane allograft that
is shelf-stable and minimally manipulated to maximize the benefits of the
amniotic membrane. SURGENEX® provides multiple sheet sizes
to provide maximum flexibility for a variety of clinical applications.
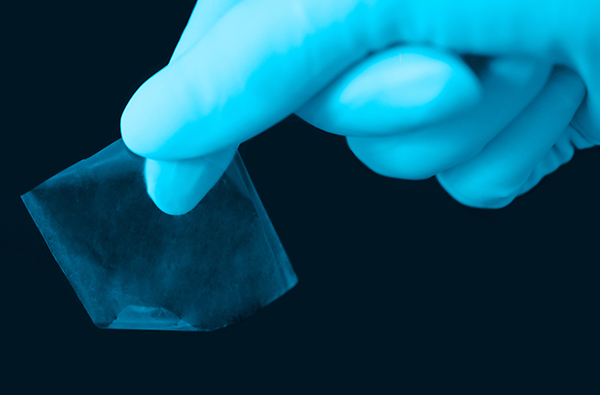
SurGraft® is single layer, giving it superior flexibility when compared to other amniotic membrane sheets on the market.

Products produced from donated human tissue are deemed qualified for acquisition by the AATB-accredited recovery agency used by SURGENEX®. Pre-screening lab tests specify the donor to be free from risk factors and active infections of applicable communicable diseases as required by the FDA.

Our process extracts the membrane from the placental tissue, leaving only the amniotic membrane layer which is then dehydrated into a sheet-like product. SurGraft‘s minimally manipulated process retains the benefits of the amniotic membrane providing clinicians with a flexible sheet for a variety of treatment applications.

SurGraft® is thoroughly tested for safety and efficacy. We pride ourselves on safety standards that exceed regulatory requirements by performing additional serological tests missed by our competitors. SURGENEX® clears allografts using a third-party serological lab, followed by quality inspections on site.